Actin is a globular protein with an ATP binding site in the center of the molecule. Termed "G-actin" the monomer will dimerize or form a trimer. This serves as a site for nucleation and further growth of the actin protofilament. Below each structure represents G-actin.
ATP is hydrolyzed immediately after the molecule is incorporated into an actin filament. The ADP is trapped in the actin filament until it depolymerizes. Then an exchange can occur.
How is the polymer formed? What ingredients do you need to make an actin filament?
G-actin forms F-actin (the filament) in the presence of ATP, Mg and K. The concentration of G-actin is also critical. Above the critical concentration Cc of G-actin, the molecules will polymerize. Below the critical concentration, the actin filaments will depolymerize. ATP hydrolysis is not required for polymerization, but it is required to promote depolymerization (if it is converted to ADP). In this regard, it behaves like microtubules and their need for GTP hydrolysis to depolymerize.

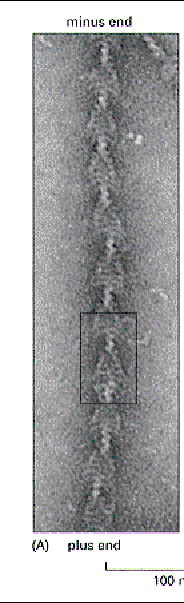
Actin filaments, like microtubules, have polarity. How is it defined in actin? What macromolecule can you use to detect polarity?
The above cartoon shows that the plus end of G-actin is the end that is opposite the cleft that holds the ATP molecule. The minus end is the opposite end. Growth and polymerization is more rapid at the plus end. If you add a solution containing myosin to actin filaments, they will "decorate the filaments" and they will be pointing in the direction of the plus end.
Describe the process of nucleation and elongation of actin filaments.
Nucleation:
Two actin molecules bind weakly, but addition of a third stabilizes the complex. This trimer then adds additional molecules and forms a "nucleation site". This is the slow, or lag phase of the polymerization process. One could add fragments of actin filaments to speed this up, in vitro. Or, key actin binding proteins may help to speed this process.
Elongation:
- Addition of actin molecules to form a long helical polymer. After a period of growth, an equilibrium phase is reached in which depolymerization controls the length as new monomers are added.
- Different actin cross linking proteins form either bundles or networks of actin filaments. Give some examples.
- Polymerization of actin filaments can occur via a network regulated by filamin. This protein works like a clip to connect the filaments at the cross-over points.
- A bundle of actin filaments might form with proteins like fimbrin, fascin, or alpha-actinin. These could fill a thin cellular process. Diagrams in the cartoon below show examples of the structural organization.

Different proteins bind actin to the plasma membrane. Give some examples.
These may vary with the cell type. Examples include alpha actinin, ankryn, spectrin, dystrophin. Many are seen in complexes that are unique to support the cells' functions. See your text for some of the cartoons and diagrams.
What are effects of cytochalasin D, latrunculin, or phalloidin on actin filaments?
These compounds bind actin. They prevent polymerization. Cytochalasin D binds to the + end of F-actin and prevents further addition of G actin. Latrunculin binds G-actin and inhibits it from adding to a filament. Phalloidin is from the poisonous mushroom (Amanita (angel of death)). It prevents actin filaments from depolymerizing. Eating quantities of raw meat may be used to treat this form of poisoning because of its high content of actin that binds to the phalloidin. Phalloidin is also used to detect actin filaments cytochemically.
What is the role of thymosin beta 4 with respect to actin filament assembly?
Thymosin binds G-actin and holds it like a buffer in case more is needed. Thus, the cell can potentially have more G-actin "in storage". This storage keeps the amount above the critical concentration, however F-actin is not formed because of the bound state of the Thymosin-actin complex.
How does profilin promote actin assembly?
Profilin stimulates assembly of actin filaments. It can complex with G-actin and attract more monomers to the + end. Thus, it may speed up the nucleation process. It may interact with membrane components in cell-cell signaling and reduce inhibitors. Or, it may be a messenger from a signalling pathway that stimulates polymerization of actin in response to a cell stimulus. It also can act as a nucleotide exchange factor, recharging the ADP actin monomers with ATP.
What are roles for severing proteins (gelsolin and cofilin) in cell motility?
The Gel state of a cytoplasm contains polymerized bundles and networks of actin. It provides stability to the leading edge of a migrating cell. However, to bring the rest of the cell along, the cytoplasm must be in the sol state...to allow flow of the contents. This is done by severing proteins that clip actin filaments, encouraging depolymerization or controlling their length. This is a calcium dependent process which is why calcium levels are higher in regions where the sol state is developing. Cofilin twists the actin filament, so it will break. AT the same time, it prevents further lengthening.
Signalling pathways will release these severing proteins to allow for the sol formation and flow of material into the cellular processes.
What are roles for capping proteins (CapZ and tropomodulin)?
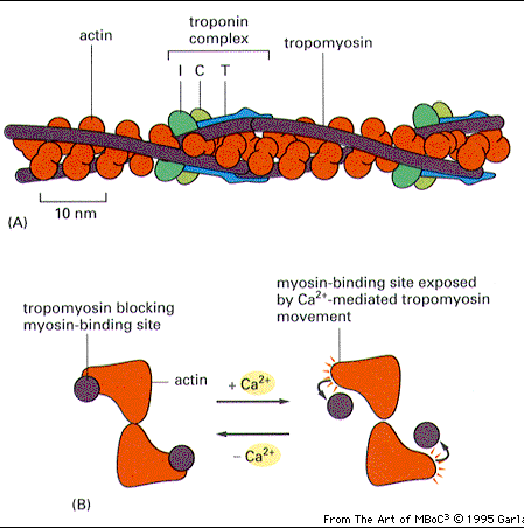
These are stabilizing molecules that interact with actin filaments. CapZ binds the + ends and prevents the addition or loss of actin subunits. Tropomodulin caps the - ends and its activity is enhanced by tropomyosin. Thus the two work together to stabilize actin. This capping is needed in sites where the actin cytoskeleton must remain stable (such as in a muscle cell.
How does the actin cytoskeleton regulate projection of cellular processes?
Projection of cellular processes is made possible by actin polymerization and formation of bundles or networks. Multiple signalling pathways provide the stimulus to accelerate polymerization. A great example is seen in the description of the Listeria bacteria transit through infected cells. These bacteria use the cell machinery to produce an actin tail that projects them through the cytoplasm rapidly. Factors like profilin can be mobilized to facilitate actin polymerization.
In characterizing different types of cell movements, what is the difference between a lammellipodium and a filopodium?
The lammillipodium is a broad shelf-like projection from a cell, such as an epithelial cell. It may be supported by a network of actin filaments and many focal adhesions. A filopodium is a thin projection or process from a cell, like a neuron or fibroblast. It is filled with a bundle of actin filaments.
What are focal adhesions and what is their significance? How do focal adhesions differ from hemidesmosomes?
Focal adhesions are spot welds that involve attachment of moving processes to the matrix. They differ from hemidesmosomes in several ways in that actin filaments attach to the specific proteins on the membrane (rather than Intermediate filaments). The attachment site is also mediated by Integrin molecules which are transmembrane receptors for specific ligands in the matrix.

Describe the basic structure and function of the cortical actin network. Study some of the examples in different cell types found in your text to see the variety in the organization.
This is the most dense concentration of actin filaments. It lies just under the plasma membrane. It interacts with a number of different proteins associated with the membrane as well as proteins in signalling pathways. It may also be traversed by myosin as vesicles are brought to the periphery for secretion. As stated earlier, it may be organized in a network or bundles, depending on the area and the needs of the cell.
At specific sites in the cell, the focal adhesions are formed, linking the cell to specific matrix proteins. It is this region that is primarily responsible for forward movement.
How is actin organized in microvilli?
Actin extends longitudinally in bundles in the microvilli. It is connected in bundles by fascin, villin, or fimbrin. Along the sides of the membrane are myosin I molecules. At the base of the microvilli are more actin filaments running perpendicularly. These form a "terminal web" and are connected by spectrin molecules. Each terminal web ends at a specialized junction called an "adherant junction". This is like a desmosome, however the filaments are actin filaments instead of intermediate filaments. The following photos and cartoons illustrate this organization.
1) This figure shows microvilli in an intestinal epithelial cell. A cross section is shown below. Each projection is filled with actin filaments.




The cartoon below shows the components of the adherent junctions.

Freeze fracture etch preparation of the base of the microvillus. This allows a view of the filaments in the terminal web (actin filament bundle).

How do myosins interact with actin? What is required for binding? What is required for movement?


Myosin is similar to kinesin in two ways. Name these similarities.

Describe an interaction in a cell where the actin moves and the myosin is stable. Describe a condition where the actin is stable and the myosin moves.
Actin moves along a stable bundle of myosin filaments in striated muscle fibers

In epithelial cells, myosin will move along a relatively stable actin cytoskeleton carrying cargo such as a vesicle. Examples are shown in the following cartoon.
How is myosin I involved with vesicular movement?
See the cartoon below for a diagram of the pathway for transport of vesicles. Most of the transport is done via microtubules. Microfilaments are involved in the final stages.

What is a sarcomere? Briefly, how does it promote muscle contraction. include roles for alpha actinin, titin, nebulin, troponin and tropomyosin.
A sarcomere is the basic contractile unit in striated muscle (found in cardiac and skeletal muscles). It is highly organized to contract with thin actin filaments sliding in along a stable myosin thick filament bundle.

Actin is found in the light band (thin filaments). Myosin is found in the dark band (thick filaments). The sarcomere extends from one z disk to another. Study the cartoon in the previous section to gain an appreciation of the 3-D view of this movement.
Actin filaments are stabilized by a backbone of tropomyosin. Troponin is spaced along the filaments as a Calcium binding site. When troponin binds calcium, the tropomyosin shifts and reveals the myosin binding site. This allows binding and movement of the thin filaments along the central myosin.
A cartoon of a sarcomere, showing the interactions is seen in the following figure.
In the thick myosin filament, titan is used to provide elasticity and stability to the structure. Nebulin is used to stabilize the thin actin filaments. Actin is attached to the Z lines by alpha actinin.
How does myosin II interact with actin in epithelial or dividing cells?

Myosin II is also found in regions depicted in the above cartoon. These are critical for motility of cell regions as well as motility of organelles.
Describe the steps involved in cell movement including: extension of the cell membrane, formation of focal adhesions, movement of the cell body and deadhesion. What are stress fibers? Compare the "chemistry of the front and rear of a moving cell".

The cartoon below shows the process.
- a) How do sol and gel states of the cytoplasm relate to cell movement?
- b) How might calcium be involved?
- c) Where are myosin I and myosin II involved?
Myosin I is in the leading edge of the cell and Myosin II is in the rear.
Calcium concentration is low in the leading edge (to prevent sol formation). It is high in the rear where the cell contents need to flow.

For more information, contact:
Gwen Childs, Ph.D.,FAAA
Professor and Chair
Department of Neurobiology and Developmental Sciences
University of Arkansas for Medical Sciences
Little Rock, AR 72205
For questions, contact this email address: